COVID-19 Stark Law Waivers
Publications - Client Alert | April 3, 2020Summary
On March 30, 2020, the Secretary of the U.S. Department of Health and Human Services (the “Secretary”) issued nationwide blanket Stark Law waivers, retroactive to March 1, 2020 (the “Blanket Stark Law Waivers”).1 The Blanket Stark Law Waivers expire after expiration of the authority of the Secretary to grant waivers for the COVID-19 outbreak in the United States. The Blanket Stark Law Waivers are set forth, categorized and explained in the Table below.
Background
As mentioned in our prior alert, on March 13, 2020, as a result of the public health emergency and national emergency declarations regarding COVID-19, the Secretary issued several waivers pursuant to Section 1135 of the Social Security Act (SSA), which waived or modified certain Medicare, Medicaid, and Children’s Health Insurance Program (CHIP) program requirements and conditions of participation, retroactive to March 1, 2020.2 These Section 1135 waivers included, among others, a waiver of sanctions under Section 1877(g) of the SSA for violations of Section 1877 of the SSA (otherwise known as the Stark Law), as determined appropriate by the Centers for Medicare and Medicaid Services (CMS).
Unlike its approach to other particular program requirements and conditions of participation, CMS did not issue additional guidance or blanket waivers of the Stark Law immediately after issuance of the Section 1135 waivers. This was consistent with CMS’s practice with respect to Stark Law waivers in prior public health emergencies, requiring providers to request waivers of the Stark Law on a case-by-case basis through a process whereby the provider was required to provide specific details concerning the actual or proposed financial relationship between the referring physician(s) and the entity furnishing designated health services (DHS).3
However, believing CMS’s existing practice was not practical or appropriate for the COVID-19 public health emergency, the American Hospital Association (AHA) asked CMS to issue a blanket Stark Law waiver, stating:
By interim final rule with comment period or exercise of its waiver authority, CMS should immediately adopt an exception to the definition of “compensation arrangement” under 42 USC 1395nn for any compensation paid to a physician or a physician’s immediate family member in return for a service necessary to the hospital’s response to the COVID-19 public health crisis.4
Blanket Stark Law Waivers
Seemingly responding to AHA’s request, the Secretary issued the Blanket Stark Law Waivers on March 30, 2020, retroactive to March 1, 2020.
CMS notes that the Blanket Stark Law Waivers may be revised from time to time as determined by CMS and that any such revisions will be posted on CMS’s website, with any revisions that narrow or terminate a blanket waiver being effective only on a prospective basis and any additional blanket waivers being effective as of the date stated in the additional waiver. Parties must meet all conditions of the Blanket Stark Law Waiver in order to rely on the waiver and each Blanket Stark Law Waiver is limited to the circumstances set forth in the waiver. If a Blanket Stark Law Waiver applies, CMS will pay claims for DHS that would otherwise violate the Stark Law.
CMS notes that parties utilizing the Blanket Stark Law Waivers are required to make records relating to such use available to the Secretary on request. CMS further notes that, although the Blanket Stark Law Waivers may be used retroactive to March 1, 2020 and do not require the submission of specific documentation or notice to the Secretary or CMS in advance of their use, CMS encourages parties to develop and maintain records in a timely manner as a best practice.
CMS sets forth the following conditions with respect to use of the Blanket Stark Law Waivers:
- The Blanket Stark Law waivers have retroactive effect to March 1, 2020, may be used nationwide and expire as after expiration of the Secretary’s authority to grant waivers for the COVID-19 outbreak in the United States.
- The Blanket Stark Law Waivers apply only to financial relationship and referrals that are related to the COVID-19 outbreak in the United States.
- Any remuneration described in the Blanket Stark Law Waivers must be directly between the DHS entity and either (i) the physician or the physician organization in whose shoes the physician stands under 42 C.F.R. 411.354(c) or (ii) the immediate family member of the physician.
- The remuneration and referrals described in the Blanket Stark Law Waivers must be solely related to “COVID-19 Purposes,” which means:
- Diagnosis or medically necessary treatment of COVID-19 for any patient or individual, whether or not the patient or individual is diagnosed with a confirmed case of COVID-19;
- Securing the services of physicians and other health care practitioners and professionals to furnish medically necessary patient care services, including services not related to the diagnosis and treatment of COVID-19, in response to the COVID-19 outbreak in the United States;
- Ensuring the ability of health care providers to address patient and community needs due to the COVID-19 outbreak in the United States;
- Expanding the capacity of health care providers to address patient and community needs due to the COVID-19 outbreak in the United States;
- Shifting the diagnosis and care of patients to appropriate alternative settings due to the COVID-19 outbreak in the United States; or
- Addressing medical practice or business interruption due to the COVID-19 outbreak in order to maintain the availability of medical care and related services for patients and the community.
The following table categorizes the Blanket Stark Law Waivers and reproduces CMS’s examples of the waivers.
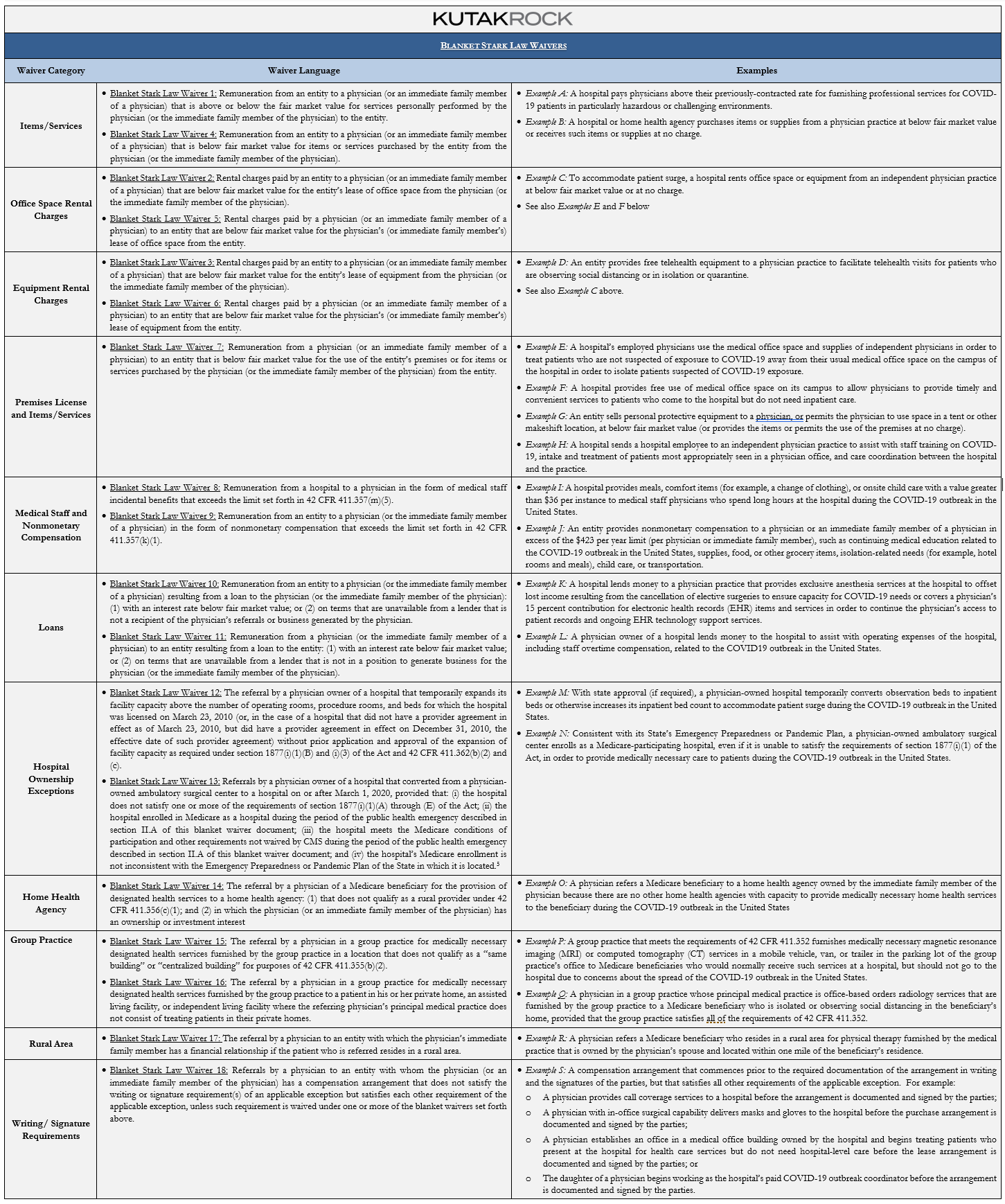
For arrangements that do not fall under the Blanket Stark Law Waivers, CMS makes clear that individual Stark Law waivers (“Individual Stark Law Waivers”) are also available and may be granted upon request. CMS outlines the following process for requesting an Individual Stark Law Waiver:
- The request must be sent via email to 1877CallCenter@cms.hhs.gov and include the words “Request for 1877(g) Waiver” in the subject line.
- All requests should include the following information:
- Name and address of requesting entity
- Name, phone number and email address of person designated to represent the entity;
- CMS Certification Number (CCN) or Taxpayer Identification Number (TIN) of the requesting entity; and
- Nature of request.
We expect that CMS will respond to a request for an Individual Stark Law Waiver within three business days of its receipt of the request, as this was the timeline set forth in the Prior Stark Law Emergency Waiver Guidance, though this is not clear.
CMS does make clear, however, that unless and until the parties to an arrangement meet the conditions of a Blanket Stark Law Waiver or have been granted an Individual Stark Law Waiver from CMS, the parties are required to comply with the Stark Law and otherwise will face sanctions, even in the context of a national public health emergency.
* * * *
Kutak Rock attorneys are actively engaged in monitoring the legal changes as a result of COVID-19, including the legislative and regulatory actions that have been taken. We have prepared a number of client alerts and special publications relating to COVID-19 which can be accessed on Kutak Rock’s COVID-19 Legal Resource Portal. In addition, determining whether an arrangement meets the requirements of a Blanket Stark Law Waiver, or requesting Individual Stark Law Waivers with respect to an arrangement, requires experienced healthcare counsel. If you have any questions about this Alert or about the impact of COVID-19 on your business, please contact a member of our national Healthcare practice group. We stand available and willing to assist you during this unprecedented time in our nation’s history.
1 A copy of the Blanket Stark Law Waivers is available here.
2 More information regarding the Section 1135 waivers can be found here.
3 Approximately a year ago, CMS released guidance setting forth its historic practice of granting Stark Law waivers on a case-by-case basis in the context of public health emergencies (the “Prior Stark Law Emergency Waiver Guidance”). The Prior Stark Law Emergency Waiver Guidance can be found here.
4 A link to the AHA letter is available here. In the letter, the AHA also requested Anti-Kickback Statute (42 U.S.C. §1320a-7b(b)) relief, stating (emphasis original):
OIG, in coordination with the Department of Justice (DOJ), should make clear by whatever means are appropriate that any transaction between hospitals, physicians and other potential referral sources, or any vendor who delivers services and supplies to hospitals, that has as its primary purpose the delivery of supplies or services necessary to the hospital’s response to the COVID-19 public health crisis will not be subject to prosecution or sanctions under the Anti-Kickback Statute or civil monetary penalties law. In addition, similar action should be taken to make clear that hospital support for patients that is necessary in responding to the COVID-19 public health crisis will not be subject to prosecution or sanctions under the Anti-Kickback Statute or civil monetary penalties law.
The OIG did post a letter on its website dated March 30, 2020 (the same date that the Blanket Stark Law Waivers were issued), though it is not clear whether the OIG’s letter was in response to the AHA letter. In its letter, the OIG notes that it will carefully consider the context and intent of the parties when assessing whether to proceed with any enforcement action with respect to any conduct during the COVID-19 emergency that may be subject to OIG administrative enforcement. The OIG letter is available here.
5 This Blanket Stark Law Waiver was issued in relation to CMS’s decision to allow currently enrolled ambulatory surgery centers to temporarily enroll as hospitals and provide hospital services to help address the urgent need for increased hospital capacity to care for patients. Additionally, under this initiative, other interested entities, such as freestanding emergency departments, can pursue enrolling in Medicare as an ambulatory surgery center and then convert their enrollment to a hospital during the COVID-19 public health emergency. More information about these initiatives, as well as CMS’s “Hospital Without Walls Initiative” is available here. Ambulatory surgery centers that wish to enroll to receive temporary billing privileges as a hospital are encouraged to call the COVID-19 Provider Enrollment Hotline to reach the contractor that serves their jurisdiction and then complete and sign an attestation form specific to the COVID-19 public health emergency. More information concerning these Provider Enrollment Hotlines is available in our previously issued Client Alert addressing Provider Enrollment Flexibilities, available here.
