COVID-19 and Quality Reporting Programs: CMS Provides Exceptions and Extensions for Reporting and Data Submission
Publications - Client Alert | March 24, 2020In furtherance of efforts to ease regulatory burdens while clinicians, providers and facilities fight the 2019 Novel Coronavirus (COVID-19), the Centers for Medicare and Medicaid Services (“CMS”) is implementing extreme and uncontrollable circumstances policy exceptions from reporting requirements and providing extensions for clinicians and providers participating in Medicare quality reporting programs. CMS is making the following exceptions to the reporting program requirements:
- For programs with data submission deadlines in April and May 2020, submission of such data is now optional, based on the facility’s choice to report.
- CMS will not use data reflecting services provided from January 1, 2020 through June 30, 2020 in calculations for Medicare quality reporting and value-based purchasing programs.
CMS notes that it is continuing to assess options to bring additional relief to clinicians, facilities and their staff. The following chart from CMS identifies the reporting program 2019 Data Submission and 2020 Data Submission updates.
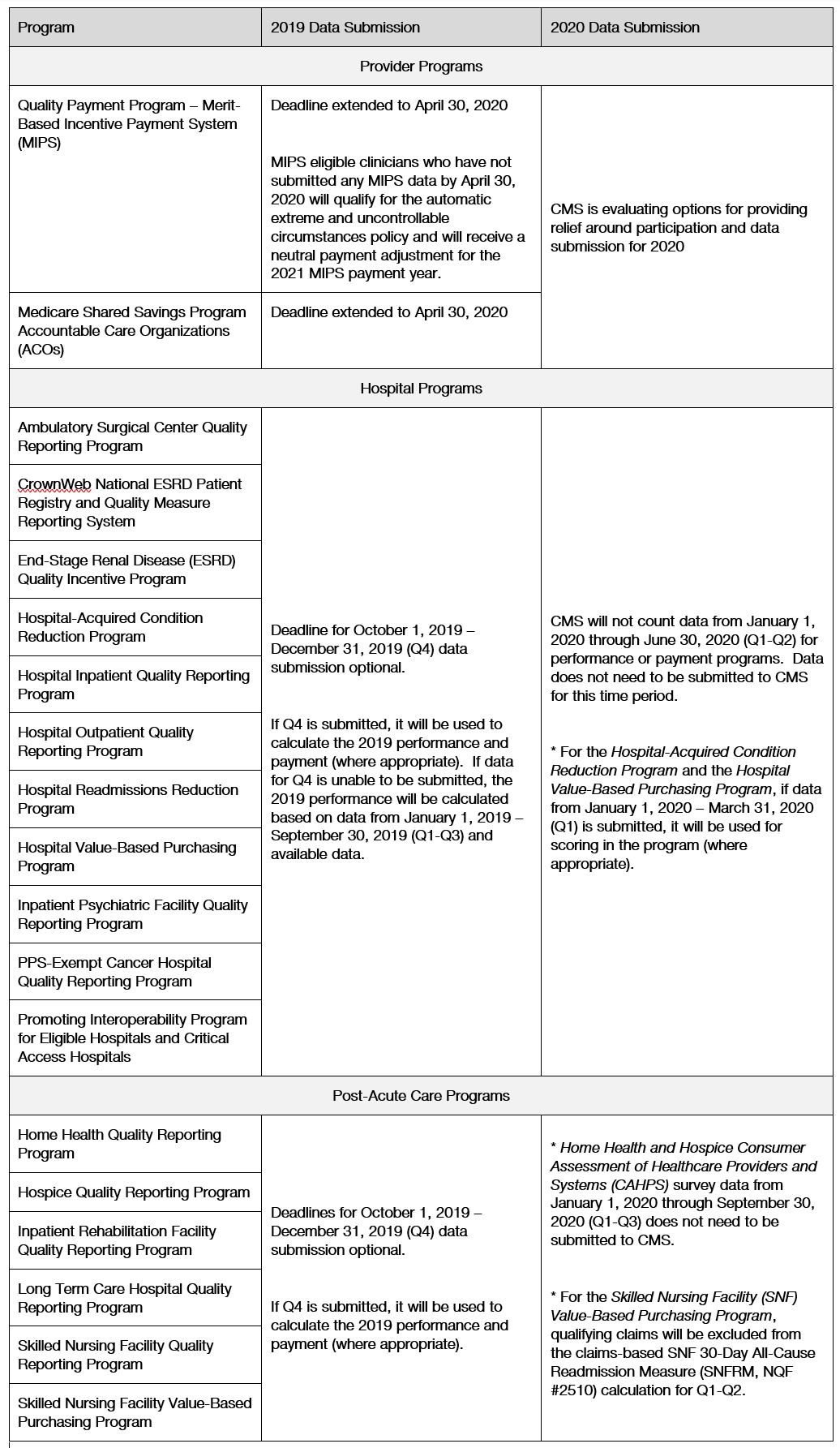
***
We anticipate that additional guidance in response to COVID-19 will be issued in the coming days and weeks, which may include additional waivers or exceptions to CMS requirements. Health care providers who are or may be affected by the consequences of the COVID-19 pandemic should monitor additional development and announcements from CMS and other federal and state agencies. If you have any questions about the Quality Reporting Program exceptions and extensions, please contact a member of our national Healthcare practice group.
